| Gary N. Cherr | Bodega Marine Laboratory |
| Rick Higashi | Crocker Nuclear Laboratory |
| Frederick J. Griffin | Bodega Marine Laboratory |
Background
The removal of volatile compounds through weathering of crude oil results in the release of low boiling point aromatic and saturated hydrocarbons. It has been thought that those components hold the greatest toxicity to marine life (Capuzzo 1987; Galt et al. 1991; Payne et al. 1991; Venkateswaran et al. 1995). Although biodegradation of crude oil can be considered a component of the weathering process, the process continues well after initial weathering and the elimination of volatile compounds has occurred. Known results of this continued microbial degradation include a measurable decrease in sediment crude oil along with a measurable organic enrichment in those sediments (Spies 1987).
As petroleum-degrading microbes are well suited to metabolizing hydrocarbons of weathered oil, enhancing such microbial biodegradation has successfully been used as an effective means of cleansing oiled sites (Mueller et al. 1992; Bragg et al. 1994). Fertilizers were employed after the Exxon Valdez oil spill in 1989 to augment the growth of microbial beach populations. Not only was there an enhanced reduction in deposited oil from the fertilized plots, there was also an enhanced loss of extractable organic matter from the remaining weathered oil (Claxton et al. 1991). Recently, studies (including our laboratory) have demonstrated that a by-product(s) of microbial degradation of artificially weathered Alaska North Slope crude oil is a ten fold increase in neutral water soluble hydrocarbons that exhibits significantly high toxicity to developing atherinid and clupeoid fish embryos (Middaugh et al. 1996, 1998). Biodegradation of crude oil occurs in regions of natural seepage (e.g. Coal Oil Point) as well as in regions of oil production and transport where elevated populations of crude oil-degrading microbes are purported to exist (Spies 1987). It can be assumed that the process of oil biodegradation at these sites is an ongoing process and that the products of that biodegradation are chronically present. The fact that the biodegradation of crude oil is now known to produce water soluble fractions that may be toxic to marine life, means there are profound implications to the biota in the Santa Barbara Channel near sites of natural oil seeps and non-catastrophic release (associated with oil production or transport). In particular, we have been interested in how select species of marine organisms tolerate chronic exposure to microbially degraded oil constituents.
Objectives of the Project
This project has been determining the effects of water soluble components of biodegraded Santa Barbara crude oil on both invertebrate and vertebrate marine organisms, and emphasizing the mechanisms utilized by some groups of organisms to tolerate relatively high concentrations of degraded petroleum compounds. We are also actively investigating the identify of the classes of compounds responsible for biological activity.
The objectives of this proposal are:
Progress to Date
While officially initiated 1 July, 1996, the project was not fully implemented until November, 1996, which was the time funding was received. Crude oil that was obtained from a Santa Barbara Channel oil platform was artificially weathered and biodegraded using microbes enriched for their oil degrading ability. The oil was artificially weathered by autoclaving at 374oC, distilled at 1 atm, and stored in aliquots at -70oC. The enriched microbe population was obtained from a seep sediment starter that was collected from Coal Oil Point in collaboration with UCSB researchers. Agar plates containing Bushnell-Haas nutrients, sea water, and 0.2% weathered oil were inoculated with the seep starter and allowed to incubate for 3 days. Further purification of oil degrading microbe colonies was accomplished by serial inoculations (2x) of growing colonies to new culture plates. Selective enrichment steps (using crude oil agar plates) have been successful since they have resulted in dramatic increases in oil degrading capabilities and have yielded a more uniform (primarily gram negative) bacterial population.
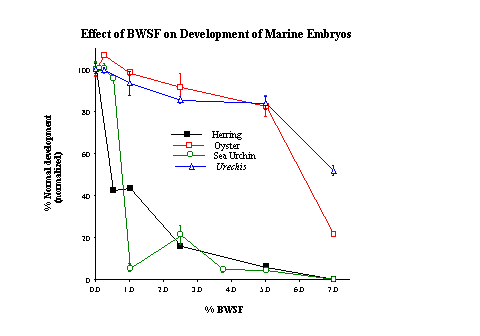
Biodegraded oil was obtained utilizing the artificially weathered oil and our enriched microbe population. Solutions of sea water containing 0.2% weathered crude oil (w/v) were incubated for 14 days at 15ºC with or without enriched cultures of microbes from Coal Oil Point. While complete emulsification of the crude oil/microbe mixture was achieved in ~10 days, the weathered crude oil/sea water mixture without microbes showed virtually no emulsification. Following incubation, both microbially degraded and non-degraded oil-water mixtures were serially filtered through 0.7 µm and 0.45 µm filters to remove particulate oil materials and microbes, aliquoted, and stored at -70oC. Aliquots were lyophilized for biological experiments as well as additional chemical fractionations. All fractions were filtered to 0.22 µm at the time experiments were initiated. Flasks containing only artificial sea water, Bushnell-Haas nutrients, and weathered oil were aerated and used as non-biodegraded controls. Aeration was utilized to insure that any volatile by-products of degradation were removed from the water. The resultant sea water from the flasks containing microbes, the biodegraded water soluble fraction (BWSF), possessed a distinctive yellow coloration and a much reduced pH (pH =5-5.7). The nondegraded flasks (minus the microbes) contained what was denoted as the nondegraded water soluble fraction (NWSF), a clear liquid with a pH of approximately 7.8. Based on the appearance of the different flasks, the crude oil-microbe treatment was almost completely emulsified as compared to the treatment without microbes. The biological effects of the sterile filtered oil/sea water solutions were assessed for their toxicity using embryos of the white sea urchin (Lytechinus anamesus), Pacific oyster (Crassostrea gigas), the fat innkeeper worm (Urechis caupo), and herring embryos/larvae (Clupea pallasi and C. harengus membras). Sea urchin and herring embryos were by far the most sensitive with adverse developmental effects being observed at concentrations of the degraded oil fraction near 0.1% (v/v) (Fig. 1).

Since the starting concentration of crude oil was 0.2% (20 ppm), the greatest amount of degraded oil in the lowest effective concentration observed in our studies to date represent approximately 20 ppb. The control (non-degraded water soluble fraction of crude oil) showed effects only at concentrations near 10%. Both Urechis and oyster embryos were significantly less affected by the degraded oil fraction, with adverse effects only being observed near concentrations of 10% and 5%, respectively. The NWSF fractions also had an adverse effect on L. anamesis development, but only at concentrations of 5-10%, which were the first concentrations where significant abnormalities were observed. The NWSF has very little or no effect on Urechis and C. gigas embryos development except near full strength concentrations.
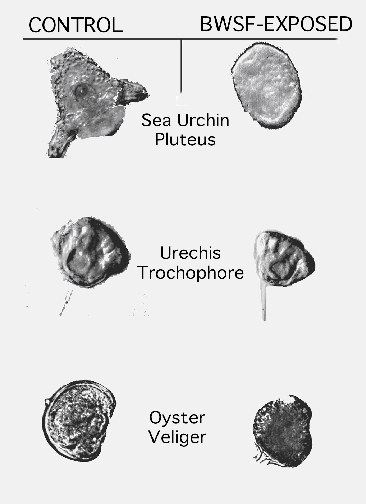
The mollusc embryos (C. gigas) exhibited a response in between those of sea urchins and Urechis. This suggests that the mechanisms involved in tolerance may also be somewhat between sea urchin embryos and Urechis embryos. A composite of normal and abnormal larvae of the three invertebrate species is shown in Figure 2.
Previous studies have shown that the neutral extractable (methylene chloride) fraction of biodegraded Alaska North Slope crude oil is responsible for toxicity to fish embryos (Middaugh et al., 1998). We have now found that for weathered and biodegraded Santa Barbara Channel crude oil, this neutral extractable fraction is not responsible for toxicity, and the more polar fraction is implicated. Work is continuing to further fractionate the polar compounds from lyophilized samples using the sea urchin embryo system to guide the fractionation efforts.
We have been focusing our efforts on the mechanisms for the different responses of L. anamesis, U. caupo, and C. gigas embryos/larvae to BWSF. We have recently shown that the degraded oil fraction is an excellent substrate for the ATP-dependent multixenobiotic resistance (MXR) transporter (a.k.a. p-glycoprotein, MDR transporter), a plasma membrane protein (ABC transporter superfamily), which is known to impart resistance to a wide range of xenobiotic chemicals throughout a number of phyla. This class of membrane transporters are well characterized in mammalian cells as they are responsible for the rapid development of resistance to chemotherapeutic drugs experienced by cancer patients. Studies since the mid 1990s have shown that invertebrates possess a similar protein which is involved in xenobiotic resistance and may be important in resistance to natural toxins as well (Kurelec, 1992, 1997). Urechis is a mud flat inhabitant whose developmental and adult life stages are exposed to a variety of natural and anthropogenic compounds that are hydrophobic. Not surprisingly, U. caupo embryos possess an active MXR transporter which protects them from environmental toxins (Toomey and Epel, 1993). Oysters from contaminated sites have been shown to have increased MXR transport activity (Minier et al., 1993), and thus this may be an inducible mechanism for resistance to organic contaminants.
To investigate whether the MXR transporter is responsible for the ability of U. caupo embryos to tolerate higher levels of BWSF as compared to L. anamesus, we used a competitive fluorescence assay which assesses the embryo's ability to export a mildly hydrophobic, non-toxic fluorescent compound (rhodamine). This can be quantitated fluorometrically in the presence or absence BWSF, which should act as a competitive inhibitor of dye transport. Our results show that BWSF does inhibit the MXR transporter's ability to export rhodamine in Urechis embryos (Figure 3).
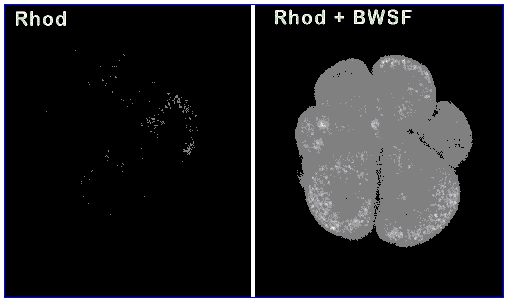
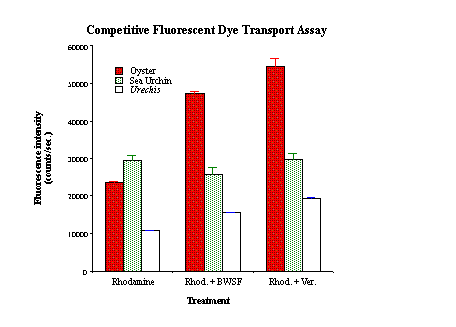
L. anamesus embryos do not possess the transporter (as has been shown in other sea urchin embryos; Toomey and Epel, 1993), and dye readily accumulates. This finding confirms that the presence or absence of the MXR transporter is likely to be the major mechanism responsible for tolerance or sensitivity of organisms to BWSF. C. gigas embryos/larvae possess the transporter but at lower levels than those of Urechis. Quantitative fluorescence data for the three organisms are presented in Figure 4.
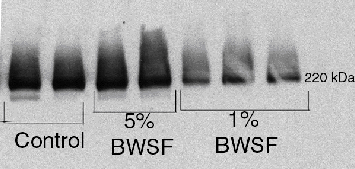
We have been investigating whether MXR transporter protein levels may be useful for assessing tolerance/sensitivity in different organisms from laboratory and, eventually field exposures. Western blot analyses of embryos and larvae are underway and preliminary data support the fluorescence data: Urechis and C. gigas embryos show the presence of the transporter, while sea urchin embryos do not. Data from adult oysters exposed to BWSF for 5 days are shown here as an example. They suggest that there may some unusual responses of the MXR protein at different concentrations (Fig. 5). Lower concentrations of BWSF appear to inhibit MXR protein expression whereas higher concentrations do not. The basis for these responses is under investigation.
Using both western blot and dye exclusion approaches, we plan to assess embryo and tissue sample from organisms collected near seep sites in the Santa Barbara Channel and compare them to the same species from sites which are not enriched for petroleum hydrocarbons. In particular, we are pursuing the question of whether sea urchins in the vicinity of seep sites express MXR protein. This would be a remarkable finding since it is currently believed that echinoderms are one of the only groups of organisms which do not express MXR protein. While the gene may be present, the protein is not readily expressed under short-term xenobiotic exposures (preliminary observations).